Question 11
(a) In an experiment to measure the specific latent heat of vapourization of water, a student places a heater in a beaker containing water. The beaker stands on an electronic balance so that the mass of the beaker and the water could be measured. The heater is switched on and readings taken every 100s when the water starts boiling.
The table below shows the readings.
Time /s |
0 |
100 |
200 |
300 |
400 |
Reading on balance/g |
203.22 |
201.62 |
199.79 |
198.26 |
196.50 |
Mass of water evaporated/g |
0 |
|
|
|
|
Energy supplied by heater/J |
0 |
|
|
|
|
(i) fill in the mass of water evaporated.
(ii) Given that the heater supplies energy at rate of 38 J/s, fill in the values of the energy supplied by the heater in 100 s, 300 s, and 400 s.
(iii) Plot a graph energy supplied on the vertical axis and mass of water evaporated on the horizontal axis, starting both axes from the origin (0,0).
(iv) Determine the slope of the graph.
(v) what does the value of the slope mean?
(b) (i) Explain what is meant by saturated vapour pressure.
(ii) State the factor that affects saturated vapour pressure.
Observation
Part (a): This Candidates performance was fair. Most candidates who attempted the question did not label the axes and were unable to correctly plot the points. Most candidates were able to correctly determine the slope but could not interpret it.
Part (b)(i): Candidates performance was fair. Most candidates were unable to explain saturated water pressure.
(ii): Few candidates were able to state that temperature is one of the factor that affect saturated vapour pressure.
The expected answer is:
(a)(i) Table of values
Time/s |
0 |
100 |
200 |
300 |
400 |
Reading on balance/g |
203.22 |
201.62 |
199.79 |
198.26 |
196.50 |
Mass of water evaporated/g |
0 |
1.60 |
3.43 |
4.96 |
6.72 |
Energy supplied/J |
0 |
3800 |
7600 |
11400 |
15200 |
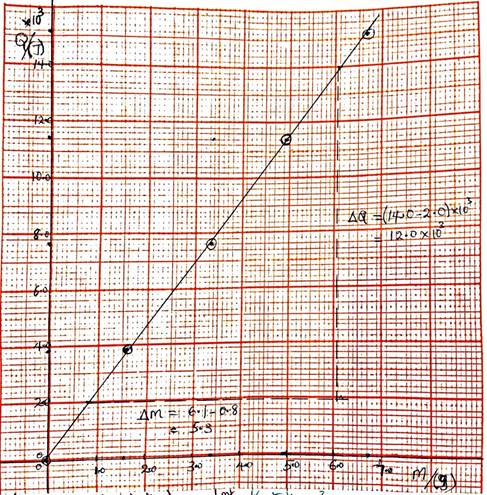
(iv) Determination of slope of graph
Slope = ![]()
![]()
= 2.26 x 103 J/g
(v) Meaning of the value of the slope of graph
2.26 x 103 J of heat energy is needed to evaporate 1 g of water at constant temperature
(b)(i) Explanation of saturated vapour pressure
At any given temperature, some energetic molecules of a liquid in a given system escape from the liquid surface as vapour and at the same time some return to the liquid. Vapour pressure is built up above the liquid in the process. When the rate of escape equals the rate of return, the vapour is said to be saturated and the pressure it exerts is called saturated vapour pressure at that temperature.
(ii) Factor that affects saturated vapour pressure
Temperature
