Question 1B
D is a solution containing 3.15 g of HNO3 in 500 cm3 of solution.
E is a solution containing 14.6 g of Na2CO3.x H2O per dm3.
- Put D into the burette and titrate it against 20.0 cm3 or 25.0 cm3 portions of E using methyl orange as indicator.
Repeat the titration to obtain concordant titre values.
Tabulate your results and calculate the average volume of D used.
The equation for the reaction is:
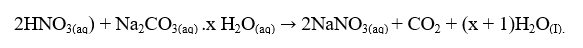
- From your results and the information provided, calculate the:
- concentration of D in mol dm-3;
- concentration of anhydrous Na2CO3 in E in mol dm-;
- volume of x in Na2CO3.xH2O.
[H=1.0; C=12.0; N = 14.0; O = 16.0, Na = 23.0]
[22 marks]
Observation
This question was on titration experiment. Majority of the candidates that responded to this question performed above average.
In part (a), majority of the candidates obtained concordant values from the titration experiment;
In part (b), majority of the candidates calculated the concentration of D in mol dm-3 and concentration of anhydrous Na2CO3 in E in mol dm-3.
The expected answers include:

