Question 1A
A is an aqueous solution of Iodine. B is 0.100 mol dm-3 sodium trioxothiosulphate (II).
- Put B into the burette. Pipette 20.0 cm3 or 25. 0 cm3 of A into a conical flask. Add B from the burette until the reddish-brown colour fades to pale yellow, then add a few drops of starch indicator to obtain a dark blue solution. Continue adding B slowly from the burette until one drop of B causes the blue colour to disappear, leaving a colourless solution.
Repeat the titration to obtain concordant titre values.
Tabulate your results and calculate the average volume of B used.
The equation for the reaction is:
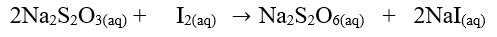
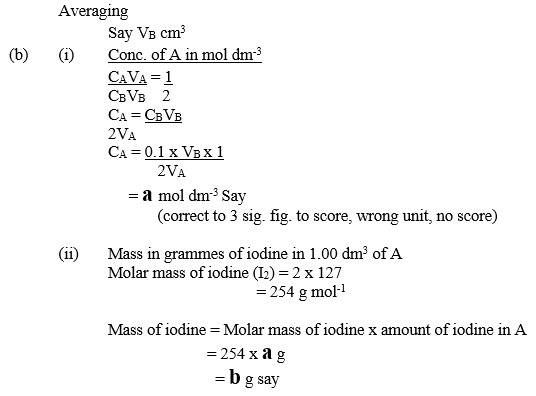
- From your results and the information provided, calculate the:
- Concentration in mol dm-3 of iodine in A;
- mass in grammes of iodine in 1.00 dm3 of A.
[17 marks]
Observation
Majority of the candidates responded to this question and their performance was above average.
In part (a), majority of the candidates carried out the titration experiment correctly;
In part (b), majority of the candidates calculated the concentration in mol dm-3 of iodine in A and mass of iodine in 1.00 dm3 of A.
The expected answers include:
(a) Two concordant titres