Question 3
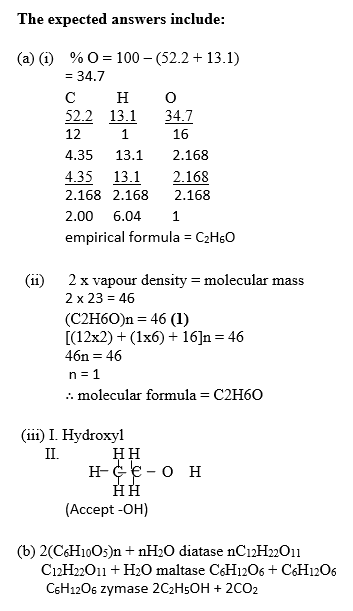
(a) A compound contains 52.2% C, 13.1 % H and Oxygen only. The vapour density of the
compound is 23.
(i) Determine its empirical formula.
(ii) Determine its molecular formula.
(iii) The compound reacts with sodium metal to produce hydrogen gas and when
warmed with acidified KMnO4(aq) gives a solution which turns from purple to colourless.
It also forms a sweet smelling liquid when heated with ethanoic acid in the presence of concentrated H2SO4.
(I) name the functional group present in the compound;
(II) draw the structural formula of the compound
[H = 1.0, C = 12.0, O = 16.0]
[10 marks]
(b) Outline the chemical equations for the production of ethanol from cooked cassava.
[6 marks]
(c) (i) Explain briefly why a piece of aluminium does not react with water.
(ii) How can a pure sample of aluminium chloride crystals be prepared from aluminium. [6 marks]
(d) Describe how water can be separated from aqueous CuSO4.
[3 marks]
Observation
Majority of the candidates did not answer this question satisfactorily, but they did well in question (a).
In part (a), majority of the candidates were able to determine the empirical formula and the molecular formula of the compound.
In part (b), majority of the candidates could not outline the chemical equations for the production of ethanol from cooked cassava.
In part (c), majority of the candidates were able to explain why a piece of aluminium does not react with water. In addition, they were able to describe how a pre sample of aluminium chloride crystals could be prepared from aluminium.
In part (d), majority of the candidates could not describe how water could be separated from aqueous CuSO4.
The expected answers include:
(c) (i) The reaction starts but the metal produces a layer of an oxide / aluminium oxide
Layer on its surface which is impermeable (to water) and stops the reaction.
(ii) Add dilute hydrochloric acid to excess aluminium filter off the excess
Metal leave filtrate in a warm place / evaporate filtrate to point of crystallization/ leave in the sun
(d) - The flask is connected to condenser and the solution is heated. The water vaporizes and is
converted to (liquid) water in the condenser.
