Question 2
(a) In an experiment, 20.0cm3 of a solution containing 4 g dm-3 of sodium hydroxide was
neutralized by 8.0 cm3 of dilute tetraoxosulphate (IV) acid:
(i) write a balanced equation for the reaction;
(ii) calculate the concentration of the acid in mol dm-3
(b) (i) State two postulates of the Kinetic theory of gases which real gases do not obey.
(ii) Explain briefly why real gases do not obey the postulates stated in 2(b)(i) [6 marks]
(c) Consider the following compound:
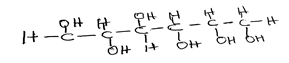
(i) name the compound;
(ii) name the two structural isomers of the compound;
(iii) state the chemical process involved in the preparation of the compound from
starch;
(iv) write the chemical equation for the steps involved in the process in 2(c)(iii);
(v) name two enzymes involved in the process in 2(c)(iii)
[10 marks]
(d) Explain briefly the term structural isomerism
[3 marks]
Observation
This question was popular among the candidates and their performance was average.
In part (a), majority of the candidates were able to write and balance the neutralization reaction between NaOH and H2SO4.
In part (b), majority of the candidates could not state two postulates of the Kinetic theory of gases which real gases do not obey, and could not explain why real gases do not obey the stated postulates.
In part (c), majority of the candidates did not answer this question, and those who did could not name the compound correctly, and few o them could state the chemical process involved in the preparation of the compound from starch.
In part (d), majority of the candidates were able to explain structural isomerism.
The expected answers include:
- (i) H2SO4 + 2NaOH → Na2 SO4 + 2H2O
(ii) Concentration of NaOH (CB)
CB = 4/40
= 0.1 mol dm-3
CAVA = 1
CBVB 2
CA x 8 = 1
0.1 x 20 2
CA = 0.1 x 20 x 1
2 x 8
= 0.125 mol dm-3
Alternative method
CB = 4/40 = 0.1 moldm-3
VB = 20/1000 = 0.02dm3
nB = 0.1 x 0.02 = 0.002 mol
∴ nA = 0.002/2 = 0.001 mol
8 cm3 of acid ≡ 0.001 mol
∴ 1000 cm3 = 0.0018 x 1000
= 0.125 moldm-3
(b) (i) - The volume of a gas is negligible compared to the total volume of the container
- There are no forces of attraction or repulsion between molecules of the gas / collision
between molecules are elastic
(ii) The volume of a gas is not negligible because at high pressure the molecules occupy
volume which is appreciable and at low temperature the intermolecular forces of
attraction and repulsion become significant
(c) (i) - Glucose
(ii) - Fructose
- Galactose
(iii) -hydrolysis
(d) - Is the existence of compounds with the same molecular formula but
different structural formulae / arrangement of the atoms / linkage of the atoms
