Question 3
3(a) Copper (II) oxide was reacted with a dry hydrogen gas as shown in the following diagram.
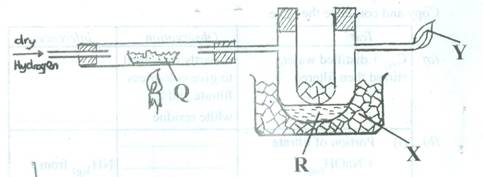
Study the diagram and answer the questions that follow.
- Identify Q, R, X and Y.
- What will be the colour change of the copper (II) oxide?
- What is the role of X? [ 7 marks]
(b) State one gas which:
(i) smells like rotten egg;
(ii) has an irritating smell like that of burning sulphur;
(iii) has yellowish green colour;
(iv) fumes with HCl gas;
(v) has reddish brown colour. [5 marks]
(c) Give one use each of the following laboratory apparatus:
( i ) volumetric flask;
(ii) wash bottle;
(iii) glass rod;
(iv) dessicator { 4 marks}
Observation
In part (a), majority of the candidates could not identify the colour change of copper (II) oxide in the diagram. They could not explain the role of X in the diagram.
In part (b), majority of the candidates could not state a gas that has reddish brown colour.
In part (c), candidates could not identify the functions of the laboratory apparatus.
The expected answers include:
QUESTION 3
- (i) Q – Copper (II) oxide
R- Water
X- Ice cube
Y- Flame of burning hydrogen
(ii) Black to reddish brown
OR changes to reddish brown
(iii) X cools / condenses water vapour formed
OR X acts as a coolant
- (i) Hydrogen sulphide / H2S
- Sulphur (IV) oxide / SO2
- Chlorine / Cl2
- Ammonia / NH3
- Nitrogen (IV)oxide / NO2
- (i) Volumetric flask: for preparing standard solutions
(ii)Wash bottle: (contains distilled water) for rinsing containers /for topping volumes
up to the mark.
(iii) Glass rod: for stirring mixtures/to transfer liquid from one container/ testing for
ammonia gas
(iv) Desiccator: for keeping substances dry
