Question 3
- (a) (i) Define the term Avogadro’s number.
- If 2.30 g of an oxide of nitrogen, contains 3.01 x 1022 molecules, calculate the
molar mass of .
(iii) Deduce the formula of
[NA= 16.02 x 1023, N = 14.0, O = 16.0]
[9 marks]
(b) (i) Describe briefly what happens when each of the following substances are added
to water:
- CCl4;
- SiCl4.
(ii) Explain briefly why the reactions in 3(b)(i)(I) and 3(b)(i)(II) are different.
[6 marks]
(c) Study the diagram below and answer the questions that follow.
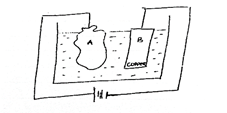
- What is the set-up used for?
- Mention two compound that could be used as electrolytes in the cell.
- Write a half-cell equation for the reaction at the anode.
- Calculate the electrochemical equivalent of copper if the cathode gained mass of 3.2 g when 50 amperes of current was passed for 5 mins 13 seconds.
[10 marks]
Observation
A fair attempt was made on the question and the performance of candidates was below average.
In part (a), majority of the candidates defined the term Avogadro’s number. However, they could not calculate the molar mass of x from the given values.
In part (b), few candidates could describe what happens when CCl4 and SiCL4 are added to water.
In part (c), majority of the candidates could not state what the set-up is used for. However, they mentioned two compounds that could be used as electrolytes in the cell. Also, they could not calculate the electrochemical equivalent of copper if the cathode gained mass of 3.2 g when 50 amperes of current was passed for 3 mins and 13 seconds.
The expected answers include:
(a) (i) Number of particles (atoms, molecules, ions etc) present in one mole of a substance.
OR
Number of particles present in 12.0 g of carbon – 12
(ii) n = N
L
moles of X = 3.01 x 1022
6.02 x 1023
= 0.05
but no. of moles = 2.30 g
Molar mass
or Molar mass = 2.30 g
No. of moles
= 2.30
0.05
= 46 g
Alternative
3.0 x 1022 molecules has a mass of 2.30 g
1 mole of a substance contain = 6.02 x 1023 molecules
6.02 x 1023 has a mass of 2.30 x 6.02 x 1023
3.0 x 1022
= 46 g mol-1
(iii) let Nx Oy be the formula of the oxide
or 14 x + 16 y = 46
x = 1, y = 2
ie. 14 (1) + 16(2) = 46
formula of x = NO2
(b) (i) I. Two layers are formed / no reaction / immiscible
II. there is a reaction / hydrolysis with steamy fumes / white
fumes
(ii) The reaction occurs by interaction of the lone pair of electrons of water binding to
the central atom (silicon or carbon) / attaching to the central atom
In silicon, the 3d – orbital is available to accept the pairs of electrons but in carbon there is no vacant orbitals for bonding
OR
Water is polar hence the reaction with SiCl4. But CCl4 is non polar hence there is no reaction
(c) (i) - Purification of metals / copper
- Electroplating
- Extraction of copper
(ii) - CuSO4
- Cu(NO3)2
- CuCl2(aq)
(iii) Cu(s) → Cu2+(aq) + 2e-(aq)
(iv) m = ZIt
t = 3 mins 13 sec
= 193 sec
Z = m
It
= 3.2
50 x 193
