Question 5
(a) (i) State two methods for the industrial preparation of hydrogen gas.
(ii) Describe briefly the laboratory preparation of hydrogen gas from the action of steam on iron.
(iii) Write a balanced chemical equation for the reaction in 5(a)(ii).
[8 marks]
(b) Describe briefly how bauxite is purified before extraction.
[6 marks]
(c) (i) State three uses of ammonia.
(ii) Outline the industrial preparation of ammonia.
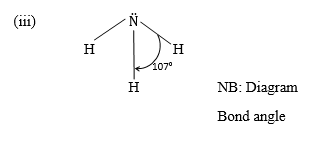
(iii) Draw and state the shape of a molecule of ammonia.
[11 marks]
Observation
This question was not well answered by candidates as majority of them did not understand the demand of the questions.
In part (a), majority of the candidates could not state two methods for the industrial preparation of hydrogen gas and could not describe the laboratory preparation of hydrogen gas from the action of steam on iron.
In part (b), majority of the candidates could not describe how bauxite is purified before extraction.
In part (c), majority of the candidates stated three uses of ammonia.
The expected answers include:
(a)(i) - from water gas/ Bosch process
- from methane
- by electrolytic method
- from catalytic cracking of petroleum products
(ii) Fill a combustion tube with some iron filing. Heat the iron filings strongly until it is red-hot generate steam by boiling water in a flask and pass the steam over the red-hot iron. Hydrogen gas would be given off. Collect the hydrogen gas formed over water.
(iii) 3Fe(s) + 4 H2O(g) Fe3O4(s) + 4H2(g)
(b) The bauxite is crushed into powder and then roasted in air. It is digested/dissolved into hot concentrated NaOH. The mixture is filtered and cooled, few crystals of Al(OH)3(s) is added. The precipitated Al(OH)3 is filtered off, it is then heated to give pure Al2O3/alumina.
(c)(i)
- Manufacture of fertilizers
- Manufacture of trioxonitrate (V) acid
- Manufacture of sodium trioxocarbonate (IV)
- Used in detergents
- Pharmaceuticals
- Liquid NH3 is used as refrigerant
- Precipitating agent
- Softening hard water
- Manufacture of explosive/dye/pesticides
- Treatment of insect stings
- Production of plastics
(ii) Dry nitrogen and hydrogen are mixed in proportion of 1: 3 respectively in volume. It is subjected to a relative high pressure of 200 – 500 atmosphere with (reduced) iron catalyst at a temperature of 350º - 500ºC